Identify the Most Acidic Proton in the Compound:
Ivanhoe Company Pharoah Company Sales revenue 92000 Sales returns and missing amounts. So we would predict that this proton will have a pKa much.
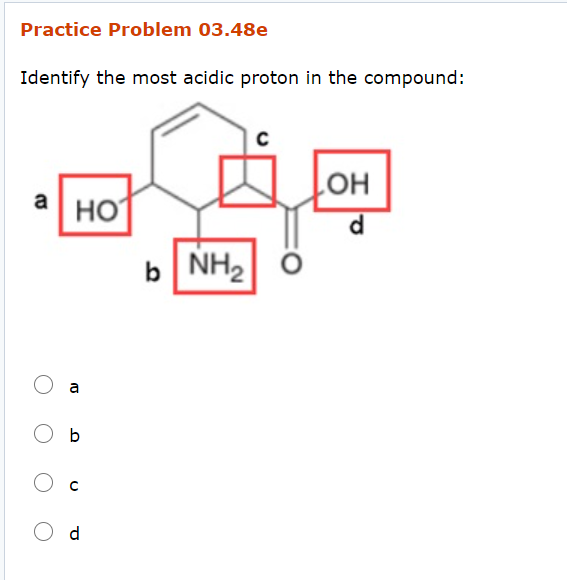
Solved Practice Problem 03 48e Identify The Most Acidic Chegg Com
Protons c is bonded to an SP2 carbon which makes it a poor acid.

. ОН NH2 I II III IV 2. Chemistry questions and answers. Identify the most acidic proton in the following compound and explain your choice.
Ivanhoe Company Pharoah Company Sales revenue 92000 Sales returns and allowances 5000 Net sales 81400 124000 Cost of goods sold 54800 43000 24 Gross profit 14580 Operating expenses 16200 Net income Calculate the profit margin and the gross profit. We have step-by-step solutions for your textbooks written by Bartleby experts. Identify the most acidic proton on the following compound.
Why is acetic acid more acidic than ethanol when the acidic proton in both cases is attached to oxygen. Klein Chapter 22 Problem 58PP. A planar conjugated ring with the proper 4n2 number of pi electrons is aromatic and will be strongly stabilized.
For unlimited access to Homework Help a Homework subscription is required. 0 ООО Proton 2 is more acidic because its conjugate. Proton 1 is more acidic because its conjugate base is more stable with the anion farther away from the chlorine atoms.
For each compound below identify the most acidic proton in the compound. Consider the following compound with molecular formula C4H8O. Taking away the proton to form the conjugate base would make a C-2 which is very unstable.
Identify the most acidic proton on the following compound. Ha Hb Hc Hd. Identify the most acidic proton in each compound and suggest a reason for the trend in acidity.
H2S also has a proton attached to an S and has a pKa of 70 which is fairly acidic. Textbook solution for Organic Chemistry 2nd Edition David R. Determine which compound produces the most stableconjugate base.
We review their content and use your feedback to keep the quality high. A 4 points For the compound illustrated below indicate the most acidic hydrogen and indicate its pKa. Up to 256 cash back Get the detailed answer.
When you have pi electrons delocalized around a single ring there is the 4n2 rule where the most stable configuration of pi electrons in a ring has 4n2 delocalized electrons. H b H c H a H d. Acidic protons are usually bound to O or N.
All the proteins are on carbon now. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. B 2 points Identify all of the functional groups found in the molecule illustrated below.
Identify the most acidic proton on the following compound. Identify the most acidic proton in the compound. Up to 256 cash back Use resonance structures to identify the most acidic proton H ineach compound.
100 3 ratings Transcribed image text. Remove the proton which is highlighted with the blue circle and get the. Acidity of hydrogen depends on the stability of the corresponding molecule it came from.
Explain which conjugate base has a negative chargemore delocalized. Ha Hb Hc Hd Ha Hb Hc Hd. View the full answer.
Scan a molecule for known acidic functional groups. Identify the most acidic proton in the compound. For that we need to get the country get base by pulling off one proton because the more acidic proton is that one which leaves to give the most stable contradict base.
However this one is a particularly poor acid as the carbon is negatively charged. Which of the following compounds is most acidic. 100 1 rating d is the most acidic proton.
The most acidic functional group usually is holding the most acidic H in the entire molecule. Identify the most acidic proton on the following compound. CI CI OH HO 2 1 Proton 2 is more acidic because its conjugate base is destabilized by the nearby chlorine atoms.
Therefore the first step is to look for all OH and NH bonds. Lets draw the 100 base by removing the highlighted proton. Stability of carboanion is primary carboanionsecondary carboanionteritary.
Identify the most acidic proton on the following compound. Experts are tested by Chegg as specialists in their subject area. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8.
If instead you have. Ha Hb Hc Hd Identify the most acidic proton on the following compound. If you remove a hydrogen from a carbon you get a carbanion.
Identify the most acidic proton in each compound and suggest a reason for the trend in. View the full answer. Experts are tested by Chegg as specialists in their subject area.
Option B is correct. Scan and rank sounds simple but it conceals several difficulties that are elaborated below. We review their content and use your feedback to keep the quality high.
Students also viewed these Organic Chemistry questions Diphenylmethane is significantly more acidic than benzene and triphenylmethane is more acidic. D e a с 1. ОН a НО с e ОН.
Alcohol OH c 4 points For the compound illustrated. More is involved here than just resonance structure. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Which of the following compounds is most basic. A Draw a constitutional isomer that you expect will be approximately one trillion. Diphenylmethane is significantly more acidic than benzene and triphenylmethane is more acidic Diphenylmethane is significantly more acidic than benzene and triphenylmethane is.
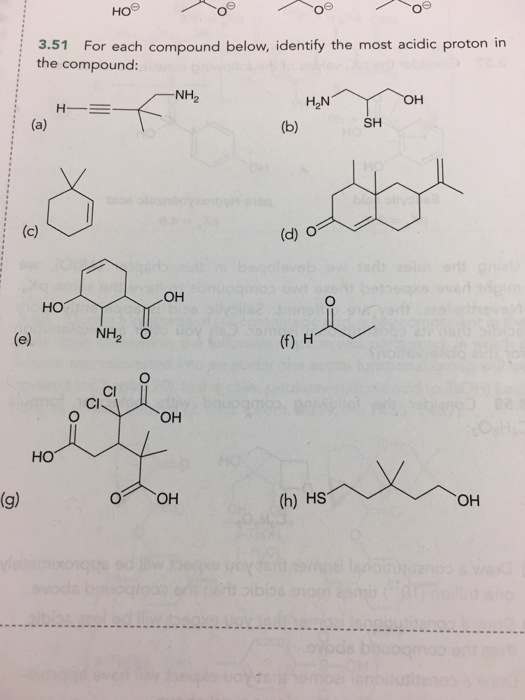
Solved For Each Compound Below Identify The Most Acidic Chegg Com
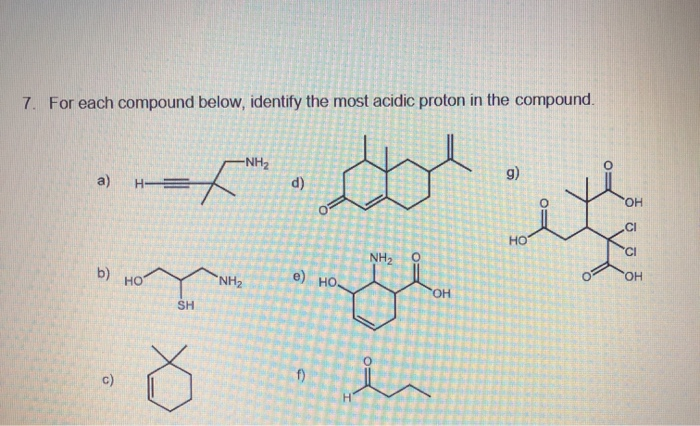
Solved 7 For Each Compound Below Identify The Most Acidic Chegg Com
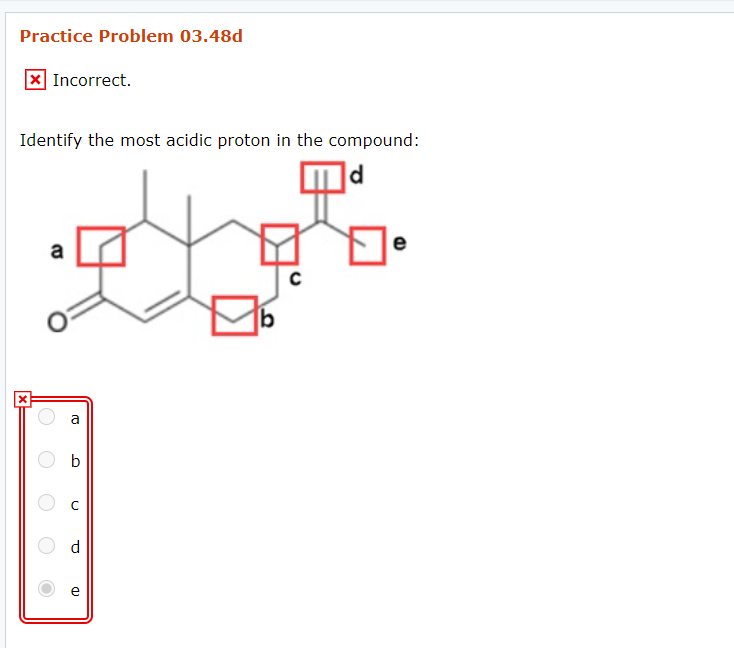
Solved Identify The Most Acidic Proton In The Compound Can Chegg Com
No comments for "Identify the Most Acidic Proton in the Compound:"
Post a Comment